When the city of Flint, Michigan, faced a water crisis in 2014, the ability to test for lead in the water and in the blood of area residents was critical. Even trace amounts could be toxic.
Fortunately, Sam Houk, professor of chemistry, had already invented an instrument that made it possible. Houk devised, demonstrated and improved an experiment called inductively coupled plasma mass spectrometry (ICP-MS), which can distinguish and measure even trace levels of elements like lead in a sample. No other method can measure such a low of concentration of elements.
Monitoring with ICP-MS
From searching for nuclear weapon activity to monitoring human health, ICP-MS detects what no other instrument can. Houk’s development of ICP-MS changed standards and methods in detecting and monitoring trace elements.
The Environmental Protection Agency (EPA) considers no amount of lead to be safe in drinking water, setting an “action level” of 15 parts per billion (ppb), the lowest concentration measurable. If more than 10 percent of samples contain more than 15 ppb, the company or government who owns the water system must implement corrosion control treatment. This concentration is only measurable with ICP-MS. In the Flint water crisis, ICP-MS measured lead in ranges such as 200 ppb to 13,200 ppb, well above the action level.
Several elements are dangerous to humans, even in low quantities, such as arsenic, mercury and cadmium. Other elements are vital for human health. The ability to measure a mix of low-quantity elements — both toxic and those necessary for health — is an important way that ICP-MS helps medical professionals.
Scientists in the Metals Lab at Mayo Clinic said that poisoning with heavy metals often occurs with generic symptoms common to numerous ailments. The results from the ICP-MS give a fuller picture for the treating physician as clinical labs offer a diagnosis (elemental toxicity or deficiency) and guide treatment decisions based upon the results obtained by ICP-MS.
Another forensic area that benefits from ICP-MS is monitoring for dirty bombs or nuclear weapon activity. Dirty bombs contain radioactive versions of elements needed by the human body, which are easily ingested upon detonation. These elements could cause cancer or other issues, but would be difficult to identify without ICP-MS.
The creation of a nuclear weapon leaves behind elements that are rarely found naturally in appreciable amounts. Detecting such trace amounts would help authorities discover the creation of a very dangerous type of bomb.
Detecting any of these radioactive elements is difficult, but ICP-MS can use samples from areas where these weapons were thought to have been created and detect the elements left behind in amounts sometimes as low as only a few hundred atoms.
How it works
ICP-MS is not the only method for detecting trace elements, but it does provide the most comprehensive picture to date. It can measure 70 or more elements down to 1 part per trillion, providing more information to be seen with fewer samples and in less time.
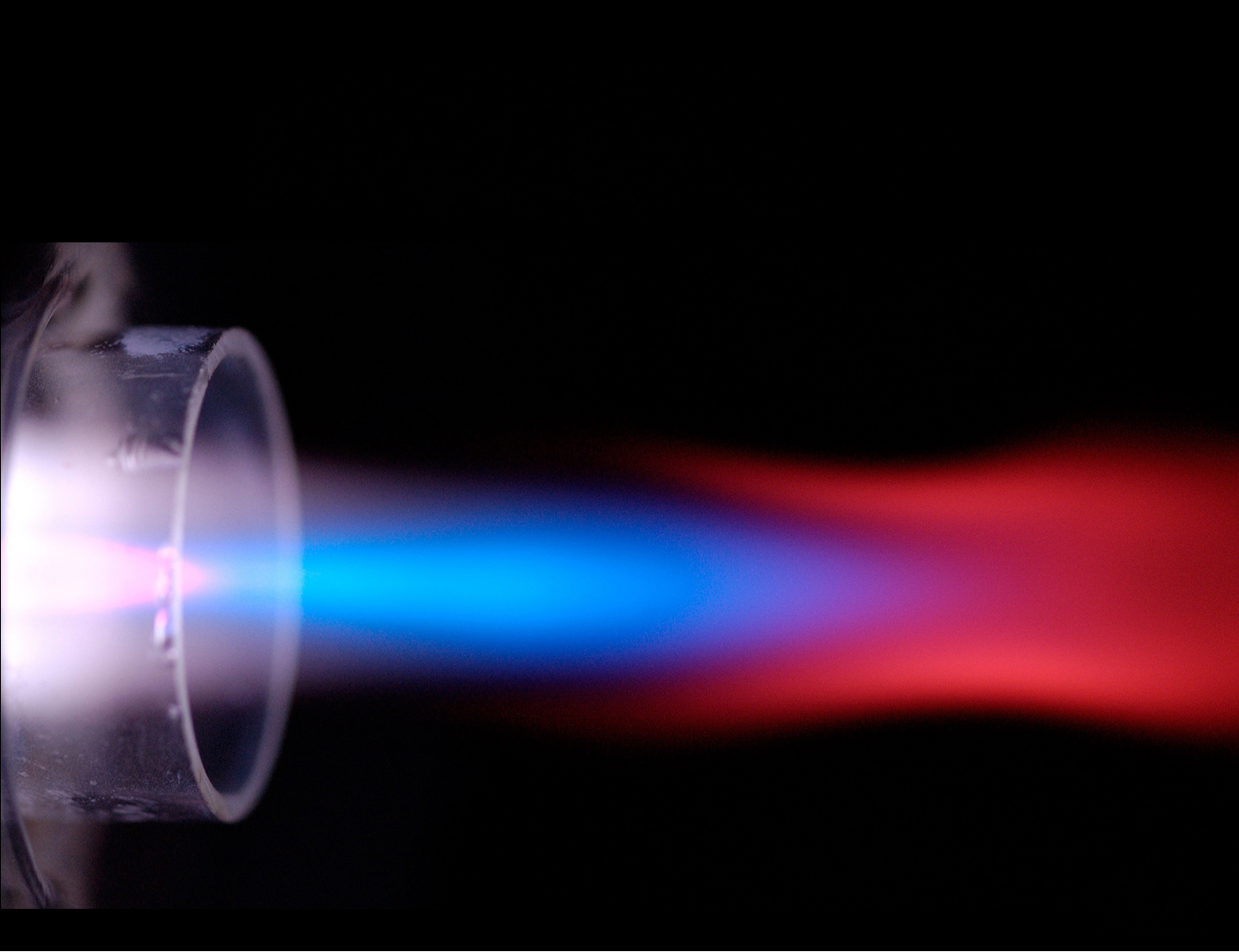
ICP-MS detection begins with injecting a sample solution into a plasma — a partially ionized gas — inside a “torch,” or glass chamber made of quartz. The torch is surrounded by a copper induction coil, which works somewhat like a microwave oven, using magnetic and electric fields to induce motion in the electrons which then collide with gas atoms. The energy transfer heats the plasma to 7,000 kelvins, hotter than the surface of the sun.
This causes the sample in the plasma to break down into atoms which are ionized, losing one electron. The ions are extracted through a metal cone at the end of the torch into a mass spectrometer, which identifies and quantifies the sample elements based on their atomic ions.
A glassblower, a machinist and a chemist
Houk became involved in the development of ICP-MS as a graduate student. Plasma and mass spectrometry were already discovered and developed but using the plasma to ionize the atoms for analysis by mass spectrometry had not been put together.
Houk first took an interest in the plasma involved in the process.
“Just watching it was fascinating,” Houk said. “Simply to look at it and realize that the temperature of it is approximately 7,000 kelvins is still fascinating to me.”
Initially he wanted to work on optical emission spectrometry with the plasma — using light emitted by the plasma to identify atoms, which was commonly done with the inductively coupled plasma. This method, however, did not measure lower concentrations of atoms.
Then he discovered papers from Alan Gray’s research at the Applied Research Laboratories in England. Gray was experimenting with mass spectrometry using a different plasma source, which sparked Houk’s idea of using inductively coupled plasma for mass spectrometry. He would just need to build instrumentation that could extract the atoms from the plasma for analysis by the mass spectrometer.
“Simply to look at it and realize that the temperature of it is approximately 7,000 kelvins is still fascinating to me.”
It took Houk two years to build a vacuum system that successfully extracted the ions into the mass spectrometer and detector, identifying the elements in the sample. To perfect the machine, he had the help of Harold Hall, a scientific glassblower in the chemistry department’s glass shop, and Kent Mogard and Tom Johnson, machinists from Ames Laboratory.
“The facilities here, and particularly the craftsman in the shop, were absolutely critical to making this work. If we didn't have machinists who could make the vacuum systems in these instruments or the glassblower who could make these torches, this would never have worked at all,” Houk said.
Fulfilling a land-grant mission
Today there are many companies that build and sell commercial versions of ICP-MS devices — more than 8,000 have been sold. Thanks to Houk’s research, the design was available for free.
Since initial publication of his design, multiple scientists worked on techniques to perform analysis with ICP-MS and have been able to couple the design to work in conjunction with other equipment. For instance, in medical monitoring toxicity may change based on what an element is chemically bound to. Because of this, ICP-MS has been paired with machines that can determine the concentration of the toxic molecule instead of just the individual atoms.
Finding trace amounts of elements may seem similar to the task of searching for a needle in a haystack. But, thanks to Houk, it is now done with an extremely sensitive, multielement metal detector.
